
PHARMACEUTICAL
- TOP
- PHARMACEUTICAL BUSINESS
PHARMACEUTICAL BUSINESS
Process Development, Test Method Development, Special Technology, Nucleic Acid Synthesis Technology
-
RESEARCH & DEVELOPMENT
Process Development, Test Method Development, Special Technology, Nucleic Acid Synthesis Technology
-
CONTRACT MANUFACTURING OF API
We have own two factories in Japan and manufacture APIs from milligram to ton scales.
-
QUALITY ASSURANCE
We have established the quality assurance and the production control system based on the cGMP.
HIGH PHARMACOLOGICAL ACTIVE SUBSTANCES

In order to respond to requirements in contract manufacturing for high potency active pharmaceutical ingredients, we have established “The facilities for high potency active pharmaceutical ingredients” which can handle manufacturing from a small scale (kg) to a large scale (ton), and the facilities have been utilized widely by our customers. MORE


NUCLEOTIDES

Recent pharmaceutical development has been focusing not only on the traditional small molecule compounds but also on middle molecule drugs which directly affect DNAs, such as biopharmaceuticals, antibody drugs and nucleic acid drugs. These new pharmaceuticals have potential to cure the incurable diseases.
In order to meet this trend, we are also actively developing methods for large-scale manufacturing of nucleic acid drugs.
MORE
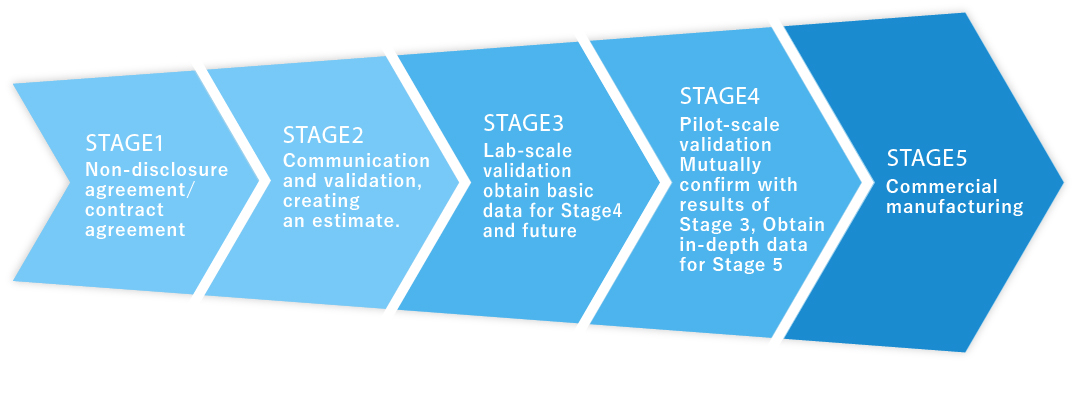
SCHEME
One-Stop-Shop(CDMO,FTE)Scheme
We provide the services from R&D of process and test method to manufacturing, based on the below scheme.
-

PHARMACEUTICAL
BUSINESSWe provide process development, method development and validation, and manufacturing services for intermediates and APIs (Active Pharmaceutical Ingredients), which have been developed by pharmaceutical companies, academia, and venture companies. Our clients can rely on our superior technologies and quality assurance system which have been developed over many years of experience in the industry.
MORE -

COSMETIC
BUSINESSWe offer sourcing, contract manufacturing and sales services of cosmetic chemicals to send beauty and moisture to customers around the world.
MORE -
CHEMICAL
BUSINESSWe supply high quality raw materials for chemicals from companies inside and outside of Japan leveraging our technologies and supply network.
MORE
CONTACT
-
+81-6-6222-0147
(Administration Division) +81-6-6201-1681
(Sales Division)Open Mon.-Fri. 9:00 am to 5:30 pm
